NCERT Solutions For Class 10 Science Chapter 2 - FREE PDF Download
NCERT Solutions for Class 10 Science Chapter 2 Acids, Bases and Salts
FAQs on NCERT Solutions for Class 10 Science Chapter 2 Acids, Bases and Salts
1. What are NCERT Solutions for Class 10 Science Chapter 2 Acids, Bases and Salts, and why are they important for CBSE 2025-26 preparation?
NCERT Solutions for Class 10 Science Chapter 2 are step-by-step answers to textbook questions provided according to the latest CBSE syllabus. These solutions help students gain conceptual clarity, follow the CBSE marking scheme, and prepare correctly for board exams as per the 2025-26 guidelines.
2. How should answers in NCERT Solutions for Class 10 Science Chapter 2 be structured according to the CBSE pattern?
Answers should follow the following CBSE methodology:
- Start with a clear stepwise explanation.
- Use relevant chemical equations for reactions.
- Highlight keywords such as pH, neutralization, and properties of acids/bases.
- Conclude with a precise statement addressing the question directly.
3. Can you explain why an aqueous solution of acid conducts electricity as per the NCERT Solutions for Class 10 Science Chapter 2?
Aqueous acid solutions conduct electricity because acids dissociate in water to produce hydronium ions (H3O+) or hydrogen ions (H+), which act as charge carriers and allow current to pass through the solution.
4. Why is it advised to add acid to water during dilution, according to Class 10 Science Chapter 2 NCERT Solutions?
Adding acid to water is recommended because the process is highly exothermic. This approach ensures that the heat generated is absorbed by the larger amount of water, minimizing the risk of splashing and accidents. Adding water directly to acid can cause violent reactions and potential burns.
5. What is a neutralization reaction as described in the NCERT Solutions for Acids, Bases and Salts Class 10?
A neutralization reaction is when an acid reacts with a base to form a salt and water. Examples:
- NaOH + HCl → NaCl + H2O
- Mg(OH)2 + 2HCl → MgCl2 + 2H2O
6. In the context of the CBSE exams, how do NCERT Solutions for Class 10 Science Chapter 2 address questions about pH and its role?
The NCERT Solutions explain that pH measures the concentration of hydrogen ions in a solution. Solutions with pH less than 7 are acidic, pH greater than 7 are basic, and pH 7 is neutral. pH helps in determining the strength and nature of acids, bases, and salts as per the board exam pattern.
7. What mistakes do students commonly make when answering acid-base questions in NCERT Solutions for Class 10 Science Chapter 2?
Common mistakes include:
- Forgetting to write balanced chemical equations.
- Confusing strong and weak acids or bases.
- Not stating observations clearly in practical-based questions.
- Neglecting correct pH values and the concept of neutralization.
8. How does the NCERT Solutions Class 10 Science Chapter 2 recommend distinguishing between acidic, basic and neutral solutions using litmus paper?
According to the solutions, red litmus paper turns blue in a base, remains red in acid or neutral solution. Further identification requires mixing with known acids or bases and observing the color change, following a logical test-tube approach as per the official CBSE method.
9. What steps should students follow to solve application-based HOTS (Higher Order Thinking Skills) questions from Acids, Bases and Salts in the NCERT Solutions?
Students should:
- Analyze the scenario or experiment carefully.
- Write related equations or chemical changes.
- Apply fundamentals like dissociation, indicators, and pH logic.
- Conclude using scientific reasoning as expected in CBSE board exams.
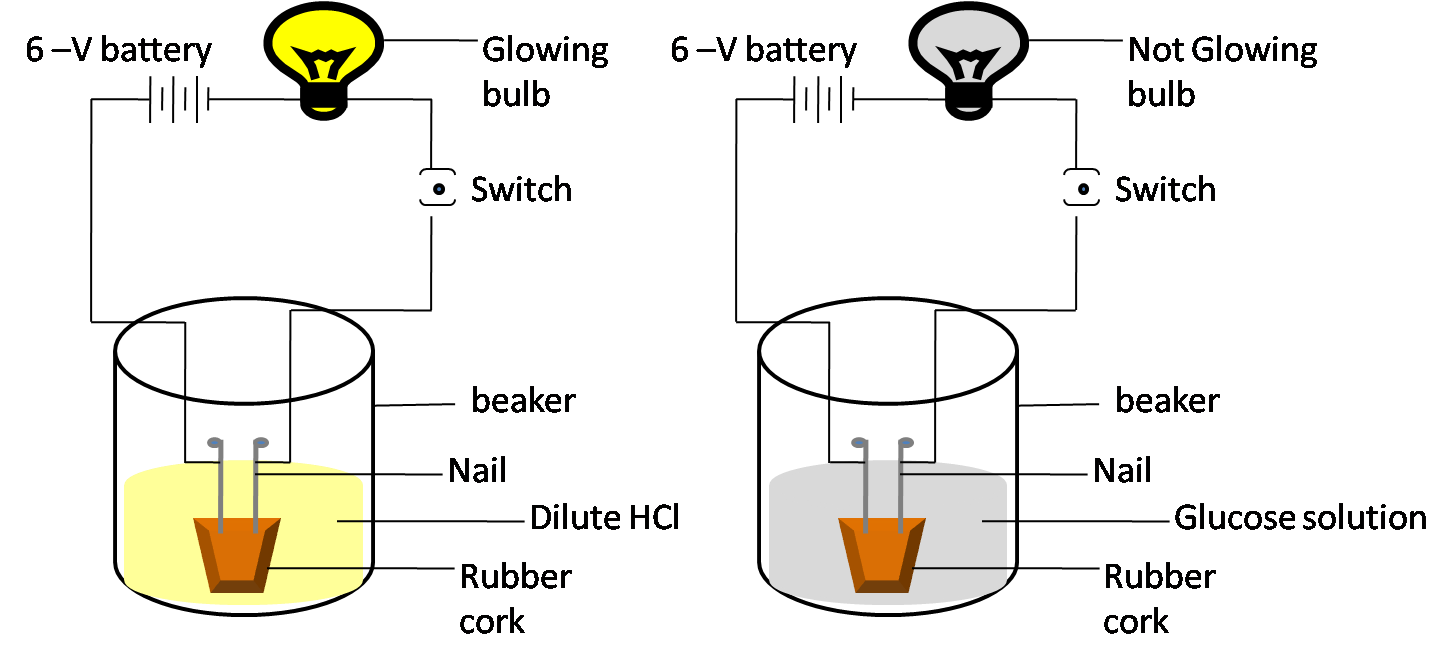
10. Why do alcohol and glucose, despite containing hydrogen, not behave as acids in aqueous solution according to NCERT Solutions for Class 10 Science Chapter 2?
Alcohol and glucose do not produce hydrogen ions (H+) on dissociation in water. Only substances that release H+ ions in aqueous medium exhibit acidic properties, which alcohol and glucose do not. Thus, they are not acids as per CBSE’s acid-base theory.
11. What is the practical significance of studying pH in daily life as taught in the NCERT Solutions for Class 10 Science Chapter 2?
Studying pH is important because it helps in understanding the acidity or alkalinity of substances such as soil, rainwater, and body fluids. Proper pH balance is crucial for agriculture (soil treatment), healthcare (antacids), and industrial processes.
12. How do NCERT Solutions for Class 10 Science Chapter 2 guide students in writing balanced chemical equations for reactions?
The solutions instruct students to:
- Identify reactants and products correctly.
- Use proper chemical symbols and formulas.
- Balance the number of atoms on both sides.
- Include states of matter (s, l, g, aq) as per CBSE 2025-26 marking scheme.
13. What is the value of practicing with NCERT Solutions for Class 10 Science Chapter 2 for CBSE board exam success?
Practicing with NCERT Solutions builds stepwise problem-solving skills, familiarizes students with the latest CBSE exam patterns, and equips them to score full marks in both theoretical and application-based questions for the 2025-26 exams.
14. How do the NCERT Solutions explain the effect of adding baking soda to milk, as per Class 10 Science Chapter 2?
Adding baking soda (sodium hydrogen carbonate) to milk makes the solution slightly basic, raising its pH. This prevents the milk from curdling quickly, as more acid is required to achieve the necessary pH for curd formation.
15. As per NCERT Solutions for Class 10 Science Chapter 2, what rules must students follow to get full marks in CBSE short and long answer questions?
Follow these rules:
- State the concept or answer directly in the first line.
- Support with chemical equations or practical observations where necessary.
- Keep explanations concise and to the point.
- Check for accuracy in terminology as per latest syllabus.














 Watch Video
Watch Video